Abstract
Sterile alpha motif and HD domain-containing protein 1 (SAMHD1), a mammalian triphosphohydrolase, plays a critical role in regulating DNA replication and damage repair. Dysregulation of SAMHD1 facilitates DNA damage-mediated cell proliferation, anti-tumor immune response and chemoresistance of cancer cells. Stimulator of interferon genes (STING) is a critical regulator of the innate immune response through the perception of DNA damage. Here, we investigated the functional significance of SAMHD1 and its regulatory effect on STING signaling.
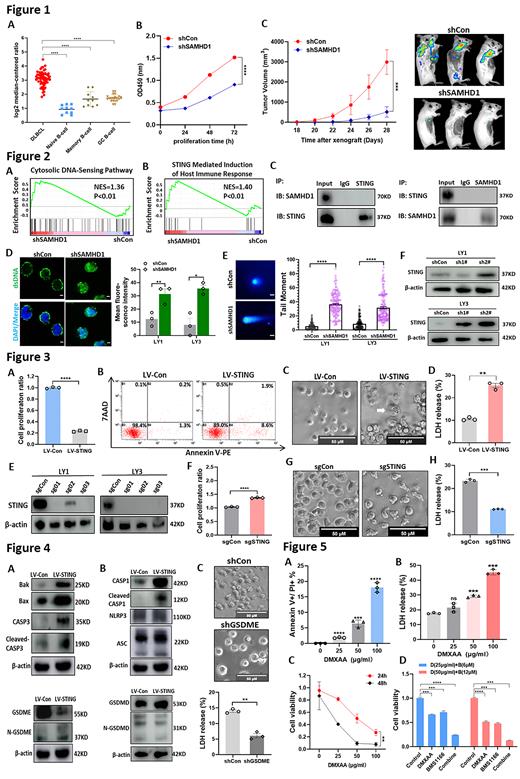
We first elucidated the expression level of SAMHD1 in DLBCL. Upregulation of SAMHD1 mRNA were identified in DLBCL cells (Figure 1A). High protein levels of SAMHD1 was validated in a cohort of newly diagnosed DLBCL patients (n=80). To further identify the biological functions of SAMHD1, SAMHD1-knockdown model was constructed in vitro. Loss-of SAMHD1 resulted not only in impaired proliferation but also in increased cell apoptosis and G0/G1 blockage (Figure 1B). To explore the function of SAMHD1 in vivo, xenograft DLBCL mice model was established. Mice bearing tumors with silenced SAMHD1 revealed delayed tumor growth and diminished tumor activity (Figure 1C).
To further explore the functional mechanism of SAMHD1 in DLBCL, we performed RNA-sequencing (RNA-seq) in LY1 cells with and without SAMHD1-knockdown. Gene set enrichment analysis (GSEA) revealed significant enrichment of the cytosolic DNA-sensing pathway, which included an elevation of STING (Figure 2A). Reactome analysis identified the enrichment of STING-mediated introduction of host immune response, suggesting the potential regulation of SAMHD1 on STING (Figure 2B). Nervetheless, co-immunoprecipitation analysis couldn't found the direct interactions between SAMHD1 and STING (Figure 2C). As a DNA damage sensor, STING activation responses to the presence of double stranded DNA (dsDNA). Our results then showed that SAMHD1-knockdown significantly induced nuclear DNA damage and cytosolic dsDNA accumulation (Figure 2D and E), thereby triggering STING activation in DLBCL cells (Figure 2F).
Next, we investigated the biological roles of STING in DLBCL. Genetic activation of STING impaired cell viability and enhanced cell death (Figure 3A-3B). To identify the form of cell death, morphological observation was performed and revealed that cell pyroptosis, represented by cell swelling with large bubbles, could be induced by elevated STING (Figure 3C). High levels of LDH were detected in STING overexpressed DLBCL cells, further verifying the emergence of cell pyroptosis (Figure 3D). To validate the involvement of STING in pyroptosis, we deleted STING by CRISPR/Cas9 genomic-editing system (Figure 3E). STING deletion not only alleviated proliferation inhibition (Figure 3F) but also restrained pyroptosis-related morphological changes and LDH release (Figure 3G and H). Expression of pyroptotic effectors were then detected to investigated the molecular mechanisms of pyroptosis. It was worth noting that the Bak/Bax-Caspase-3 mediated gasdermin E (GSDME), rather than ASC/NLRP3/Caspase-1 mediated GSDMD, was activated in STING overexpressed DLBCL cells (Figure 4A and B). Furthermore, silencing of GSDME significantly inhibited pyroptosis-induced morphological changes and LDH release (Figure 4C).
The anti-tumor effects of STING agonists in DLBCL were further explored. Treatment of DMXAA, a STING agonist, significantly promoted pyroptosis in DLBCL cells (Figure 5A-5B), further resulting in suppressed proliferation in time- and dose-dependent manners in vitro (Figure 5C). Besides, DMXAA also contributes to the upregulation of immune checkpoints, especially programmed cell death protein 1 (PD-1). We further detected the synergistic effect of DMXAA and PD-1 inhibitor (BMS1166). Of note, combination of DMXAA and BMS1166 remarkably produced an enhanced anti-tumor effect on DLBCL cells in vitro (Figure 5D).
In summary, our present study firstly demonstrates that SAMHD1 functions as an oncogene in DLBCL by inhibiting DNA damage induced STING activation. Intrinsic STING induces cell pyroptosis in DLBCL cells via activating the Bak/Bax-Caspase3-GSDME pathway. Moreover, investigation of drug combination highlights the synergistic effect of STING agonist and PD-1 inhibitor, providing a novel option for improving therapeutic effects of anti-PD-1 treatment in DLBCL.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal